All the facts about PerioChip.
What Is Periochip
PerioChip is a second line treatment in the care and maintenance of Periodontal Disease. It comes in the form of an innovative, easy to insert biodegradable chip containing 2.5 mg of chlorhexidine digluconate – an ideal non-antibiotic adjunct treatment to reduce pocket depth in adults with periodontitis.
It has been demonstrated clinically to be effective and well tolerated for periodontal pockets with a depth of 5 mm or more.
The biodegradable gelatin matrix contains 2.5 mg of chlorhexidine digluconate which is released slowly over a period of 7 days.
A high local concentration of chlorhexidine eliminates 99.9%. of bacteria in the pocket and suppresses the early recovery of the periodontal pathogenic biofilm for up to 11 weeks.
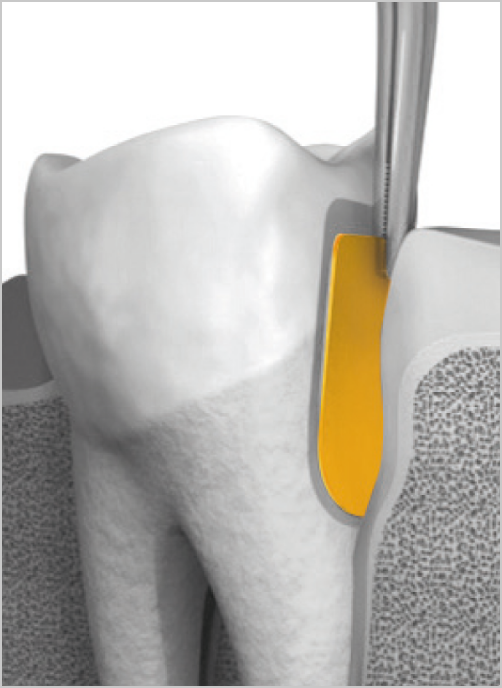
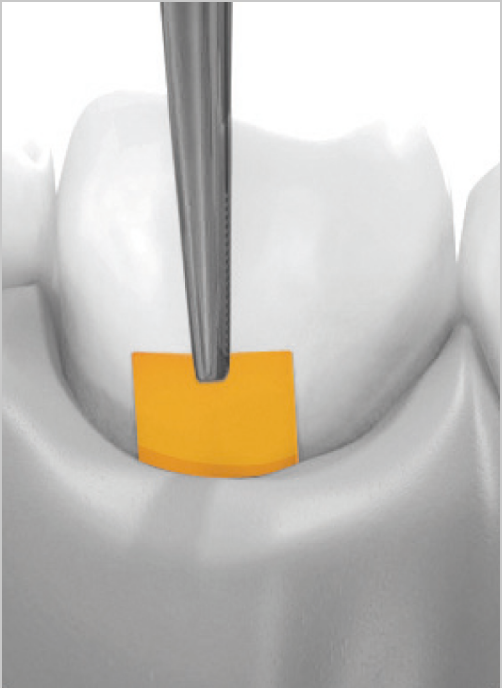
The gelatin matrix makes PerioChip strong and its unique size and shape enable easy insertion with forceps after routine root surface debridement (RSD).
PerioChip should be used as part of a medium to long term periodontal treatment plan. Give your patients the benefits of an easy, local non-antibiotic solution that is well tolerated and clinically effective.
- Indicated for pocket depth (PD) ≥ 5mm
- Significantly more effective than RSD alone
- 2.5 mg of chlorhexidine digluconate (36%)
- Wide spectrum of antimicrobial activity
- Free circulation of chlorhexidine digluconate throughout pockets
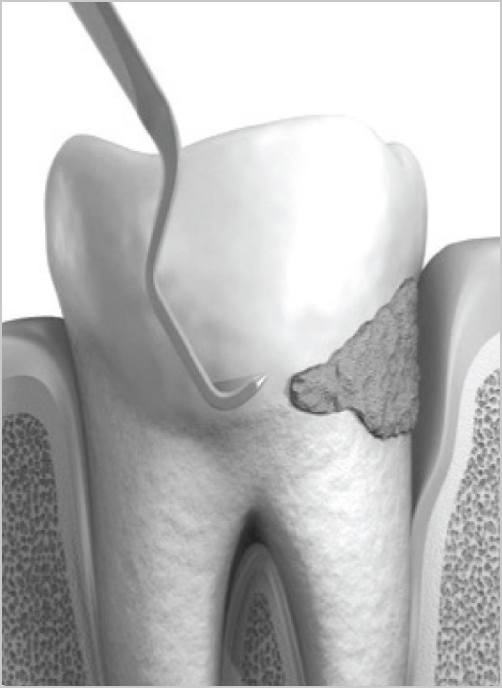
- Dissolves within 7 to 10 days, thus does not need to be removed.
- Weight: 7.4 mg
- Dimensions: 4 mm x 5 mm x 0.35 μm
- Active ingredient: 2.5 mg chlorhexidine digluconate.
- Release: Slow release over 7 to 10 days